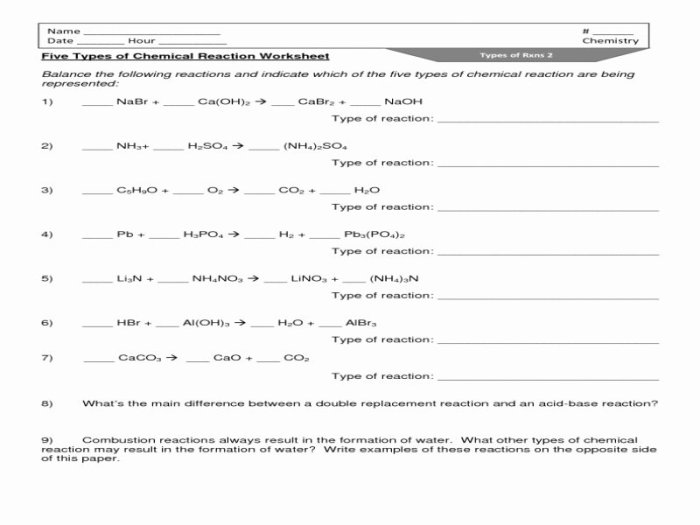
The classification of chemical reactions worksheet serves as an invaluable tool for students and educators alike, providing a comprehensive understanding of the fundamental principles governing chemical reactions. This worksheet explores various reaction types, delves into the intricacies of balancing chemical equations, and empowers learners to predict reaction products.
Its practical applications extend far beyond the classroom, impacting industries, technology, and our daily lives.
As we delve into the intricacies of chemical reactions, we will uncover the mechanisms by which atoms and molecules rearrange themselves, forming new substances with distinct properties. Through engaging examples and interactive exercises, this worksheet fosters a deep understanding of the underlying concepts, empowering learners to confidently navigate the complexities of chemical reactions.
Classification of Chemical Reactions: Classification Of Chemical Reactions Worksheet
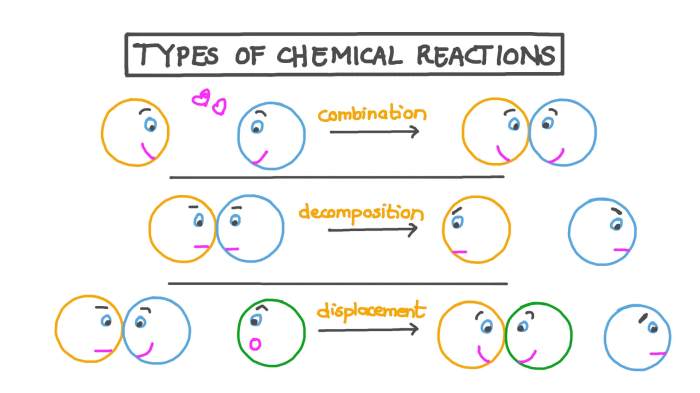
Chemical reactions can be classified into several types based on their characteristics. Each type of reaction has its own unique set of features and mechanisms. Understanding the different types of chemical reactions is essential for comprehending the behavior of chemical systems and predicting the outcomes of reactions.
Types of Chemical Reactions, Classification of chemical reactions worksheet
- Combination reactions: Two or more reactants combine to form a single product.
- Decomposition reactions: A single reactant breaks down into two or more products.
- Single-displacement reactions: One element replaces another element in a compound.
- Double-displacement reactions: Two compounds exchange ions to form two new compounds.
- Combustion reactions: A substance reacts with oxygen to produce heat and light.
| Type of Reaction | Description | Example |
|---|---|---|
| Combination | Reactants combine to form a product | 2Na + Cl2 → 2NaCl |
| Decomposition | Reactant breaks down into products | 2H2O → 2H2 + O2 |
| Single-displacement | Element replaces another in a compound | Fe + CuSO4 → FeSO4 + Cu |
| Double-displacement | Compounds exchange ions | NaCl + AgNO3 → AgCl + NaNO3 |
| Combustion | Substance reacts with oxygen | CH4 + 2O2 → CO2 + 2H2O |
Common Queries
What are the main types of chemical reactions?
The primary types of chemical reactions include synthesis, decomposition, single displacement, double displacement, and combustion reactions.
How do I balance chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each reactant and product to ensure that the number of atoms of each element is equal on both sides of the equation.
Can I predict the products of a chemical reaction?
Yes, it is possible to predict the products of a chemical reaction based on the reactants involved and the type of reaction taking place.