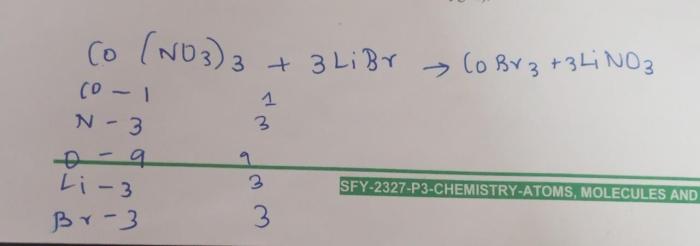Chemistry moles packet answer key – Welcome to the ultimate guide to chemistry moles! This comprehensive answer key unlocks the secrets of this fundamental concept, providing you with a clear and engaging overview of its applications and significance.
From understanding the basics of mole conversions to delving into the intricacies of stoichiometry, this answer key is your indispensable companion on your journey to mastering chemistry.
Definitions and Concepts: Chemistry Moles Packet Answer Key

The mole is the fundamental unit of amount in chemistry. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
Moles are used in stoichiometric calculations to determine the quantitative relationships between reactants and products in chemical reactions. The mole concept also provides a link between the mass and volume of a substance through molar mass and molar volume.
Mole Conversions
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol). To convert between moles and mass, we use the following formula:
- Mass (g) = Moles × Molar mass (g/mol)
- Moles = Mass (g) / Molar mass (g/mol)
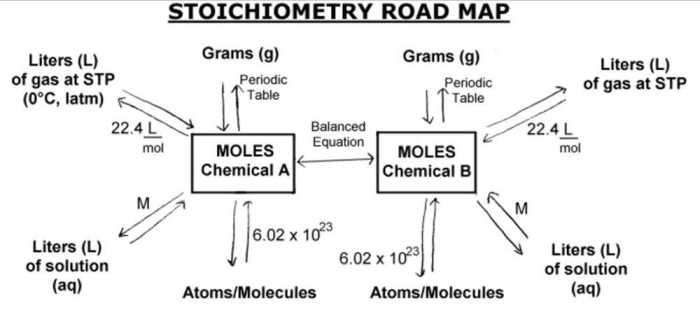
Stoichiometry and Mole Ratios
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Mole ratios are used to determine the stoichiometry of reactions and to balance chemical equations.
To use mole ratios, we first need to determine the balanced chemical equation for the reaction. The coefficients in the balanced equation represent the mole ratios of the reactants and products.
Limiting Reactants and Excess Reactants, Chemistry moles packet answer key
In a chemical reaction, the limiting reactant is the reactant that is consumed completely. The excess reactant is the reactant that is left over after the reaction is complete.
To determine the limiting reactant, we compare the mole ratios of the reactants to the coefficients in the balanced chemical equation. The reactant with the smallest mole ratio is the limiting reactant.
Solution Concentration
The concentration of a solution is a measure of the amount of solute dissolved in a given amount of solvent. There are several different units of concentration, including molarity, molality, and ppm.
To calculate the concentration of a solution, we use the following formula:
- Concentration = Moles of solute / Volume of solution (L)
Applications of Mole Concept
The mole concept has a wide range of applications in various fields, including medicine, environmental science, and engineering. For example, moles are used to:
- Determine the dosage of medications
- Monitor pollution levels
- Design chemical processes
Common Queries
What is a mole in chemistry?
A mole is a unit of measurement used to quantify the amount of a substance. It represents the amount of a substance that contains as many elementary entities as there are atoms in 12 grams of carbon-12.
How do I convert between moles and mass?
To convert between moles and mass, you can use the molar mass of the substance. The molar mass is the mass of one mole of the substance, expressed in grams per mole.
What is the relationship between moles, mass, and volume?
The relationship between moles, mass, and volume is given by the following equation: moles = mass / molar mass – volume – density.